
From raw ore to valuable manganese products, this facility showcases the intricate interplay of machinery, chemistry, and engineering. Manganese Powder Processing Plant remains paramount, Extracting this metal from its ores is a process that involves an intricate dance of physical, thermal, and chemical techniques.
24 Online Service

Its presence has shaped industries, from steel production to battery manufacturing, making it a crucial resource for modern society. This versatile metal is found in various mineral forms, each with distinct characteristics, and is distributed across the globe in intriguing geological settings.
Manganese ores are classified based on their mineral composition and physical attributes. These ores often occur in complex combinations and can vary in color, texture, and mineral structure. Three prominent types of manganese ores commonly found are pyrolusite, psilomelane, and rhodochrosite.
Pyrolusite is perhaps the most recognized manganese ore due to its black, granular appearance. Composed primarily of manganese dioxide (MnO2), pyrolusite is a major source of manganese and finds extensive use in the steel industry. Its high manganese content, usually exceeding 63%, makes it an ideal candidate for steelmaking, where it aids in removing sulfur and oxygen impurities during the smelting process.
Unlike pyrolusite, psilomelane is a complex mixture of manganese oxides that can include varying amounts of water, barium, potassium, and other elements. This mineral’s appearance ranges from dark brown to black, often with a botryoidal or stalactitic structure. Psilomelane’s composition makes it less suitable for steel production but valuable in other applications, such as pigments, ceramics, and even as an ore of barium.
Unlike the dark-hued ores mentioned earlier, rhodochrosite stands out with its striking pink to red coloration. Comprising manganese carbonate (MnCO3), this ore is aesthetically pleasing and holds value as a collector’s item. While not a primary source of manganese for industrial purposes due to its lower manganese content, rhodochrosite is an important mineral for studying geological processes and is sometimes used as a gemstone.
Manganese deposits are distributed across the world, often concentrated in specific regions that have experienced the right geological conditions. Besides the Kalahari manganese fields in South Africa, which account for a substantial portion of global manganese production, other notable manganese deposit locations include:
This region hosts vast sedimentary manganese deposits that have played a crucial role in Eastern Europe’s manganese supply.
China is a major producer of manganese, with large deposits located in these provinces. The deposits are primarily of sedimentary origin.
India possesses substantial manganese deposits, with Balaghat and Nagpur districts being prominent sources.
Gabon is home to the Transvaal Supergroup deposits, where high-grade manganese ores are found in sedimentary rocks.
Brazilian deposits are known for their manganese oxide ores, particularly pyrolusite and cryptomelane.

Its abundance in the Earth’s crust makes it a valuable resource, but obtaining this vital metal requires a series of intricate extraction processes. These techniques, ranging from beneficiation processes to pyrometallurgical and hydrometallurgical methods, are meticulously chosen based on factors like ore type, grade, and intended use.
The journey to extract manganese begins with the physical treatment of mined ores. In many cases, the first step involves crushing the raw material into smaller pieces, facilitating subsequent processing. Following this, the ore undergoes washing to remove impurities and increase its overall purity. Water is often used to separate lighter gangue materials from the denser manganese ore.
This ancient yet effective technique exploits the varying densities of different materials to separate them. Gravity separation is employed to separate manganese ore from gangue materials by utilizing the force of gravity. This process is particularly useful when the ore particles have different densities, allowing for efficient separation without the need for chemicals.
Leveraging the magnetic properties of manganese and other minerals, this method employs powerful magnets to separate magnetic materials from non-magnetic ones. This technique is particularly effective for separating ferromagnetic materials, including manganese, from non-ferromagnetic gangue materials.
Roasting involves heating the ore in the presence of air to drive off volatile components and convert certain compounds into more desirable forms. This process can be used to convert manganese oxides into manganese dioxide, a key component in batteries and various chemical applications. The controlled application of heat in roasting is crucial to achieving the desired chemical transformations without causing excessive loss of the target metal.
Smelting is a high-temperature process where the ore is heated along with reducing agents to extract the metal. In the case of manganese, carbon-rich materials like coke are used as reducing agents to separate the metal from its oxide compounds. Smelting is commonly used in the production of ferromanganese, an alloy containing both iron and manganese, which is essential for steel production.
Once the metal is extracted through smelting, refining is often necessary to achieve the desired purity level. Refining processes involve removing impurities and adjusting the composition to meet specific requirements. In manganese refining, techniques like electrolytic refining or zone refining may be employed, depending on the level of purity required for the intended application.
Hydrometallurgical processes involve using liquids, usually acids or bases, to dissolve the metal from the ore. Leaching is a common method used to extract manganese, especially from oxide ores. In this process, a suitable acid, often sulfuric acid, is used to dissolve manganese oxide minerals, leaving behind impurities. The resulting solution can then be further processed to recover the manganese.
Solvent extraction is employed to separate metals from solution using selective solvents. This method is often used to refine manganese-containing solutions obtained from leaching processes. Through a series of extraction and stripping steps, the target metal can be concentrated and separated from other elements, yielding a higher-purity product.
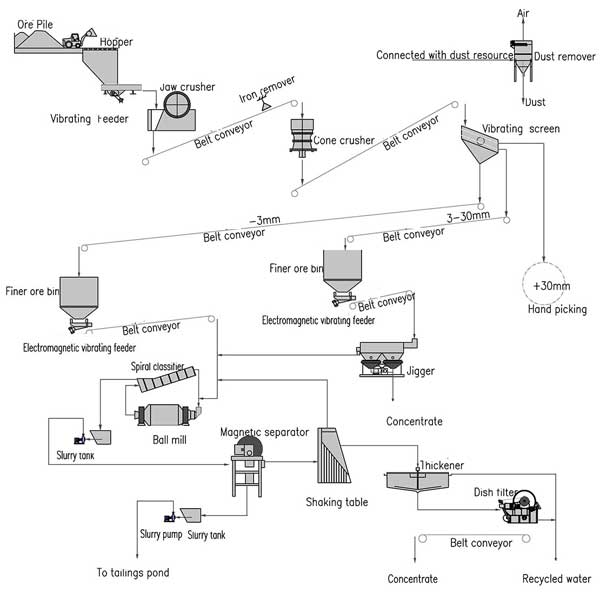
This unheralded metal plays a pivotal role in a plethora of applications, from steel production to the manufacture of batteries. At the core of this transformative journey is the Manganese Powder Processing Plant, a remarkable facility that takes raw manganese ore and metamorphoses it into valuable manganese products.
The journey from raw ore to these refined products is a complex one, requiring sophisticated machinery and processes. This is where the Manganese Powder Processing Plant steps in, serving as the cornerstone of this metamorphic process.
At the heart of the Manganese Powder Processing Plant lies a series of meticulously designed components and machinery, each contributing to the evolution of raw ore into valuable manganese products.
The journey begins with the crushing and grinding of raw manganese ore. This crucial step reduces the size of the ore, facilitating further processing. High-speed crushers and grinding mills play a pivotal role in breaking down the ore into smaller, manageable particles.
The fragmented ore is then screened and classified according to particle size. This sorting process ensures that particles are of uniform size, allowing for consistent processing downstream.
Once classified, the ore undergoes leaching, a chemical process that separates valuable manganese compounds from impurities. Through the use of reagents, solvents, and advanced separation techniques, the ore is purified, ready for the next phase.
In certain cases, electrolytic methods are employed to further refine manganese compounds. Electrolysis involves the use of electrical currents to drive chemical reactions, resulting in the deposition of high-purity manganese metal.
The purified manganese compound, now in a concentrated form, is subjected to drying and powdering. This step prepares the material for subsequent applications, whether it be in steel production, battery manufacturing, or other industrial processes.
The final step involves packaging the processed manganese into suitable containers, ready for distribution to industries that rely on this versatile element.
Beyond the mere mechanics, the Manganese Powder Processing Plant is emblematic of industrial innovation and sustainability. With an increasing emphasis on eco-friendly practices, these plants are designed to minimize waste generation and optimize resource utilization. Advanced technologies such as automated control systems, energy-efficient machinery, and waste recycling contribute to reducing the ecological footprint of the plant.
Our Projects
Copyright © ZENITH, All Right Reserved.
